Background:
Hematogone (htg) counts are usually low in bone marrows but often increase in reactive or neoplastic conditions. When increased, htgs resemble leukemic-blasts in B-lymphoblastic leukemias (B-ALL) phenotypically. The differentiation between htgs and leukemic-blasts is critical as hematogone counts in marrows with reactive conditions may increase around or beyond B-ALL leukemic-blast diagnostic level and, conversely, leukemic-blast counts may be low in some B-ALL marrow samples. Additionally, current hematogone enumeration by the gating approach is operator dependent.
Objective:
To enumerate htgs and differentiate htgs from leukemic-blasts using machine-learning (ML) models.
Research Methods:
Cases with reported htgs (CReH), B-ALL, and controls (548, 124, and 771 marrow samples, respectively) were selected using key word search from historical flow cytometry (FC) results. Datasets for analysis were from FC-panel: FS_SS_CD5_CD10_CD20_ CD19_CD45.
Three preliminary models, the htg enumeration model (HEM), immature cell enumeration models for B-lineage (IEM_B), and htg similarity model (HSM), were used (data not published). HSM classified a given htg-population or leukemic-blast-population into three categories: Htg-p (htg-population), Borderline, and non-Htg-p.
Results:
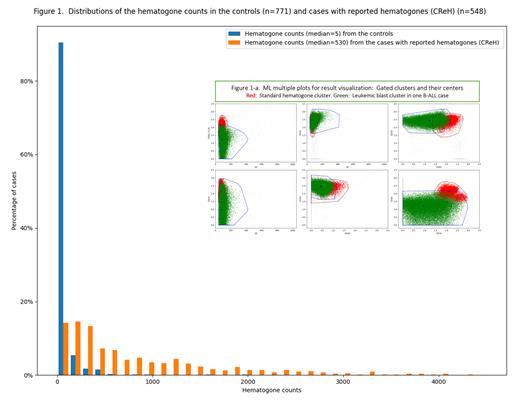
HEM distinguished CReHs with median 530 from the controls with median 5 (figure 1). Only six (<1%) of the controls had >500 htgs.
IEM_B was used for separating hgt-populations in CReHs or leukemic-blast-populations in B-ALL cases from other cell populations. HSM classified 100 (95.2%) out of 105 hgt-populations from CReHs (≥1000 htgs) as Htg-p, and, due to fluorescence intensity downward shift, 4 as non-Hgt-p and 1 as Borderline. Conversely, HSM classified 113(92%) out of 123 leukemic-blast-populations from B-ALL as non-Hgt-p, 6 (5%) as Hgt-p and 4 (3%) as Borderline. Multiple scatter plots (figure 1a) illustrate two-dimensional distributions of a leukemic cluster classified as non-Hgt-p in a B-ALL case compared with those of a standard hematogone cluster.
Conclusion:
In an experimental setting, HEM is an efficient/high-throughput alternative for hgt-enumeration with high-consistency and is operator-independent. In the majority of B_ALL cases, HSM was able to differentiate leukemic-blast-populations from htg-populations. Future models will use additional markers, including leukemia-specific ones, to increase models' specificity.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal